Summary of Pact Management and two initial proposed studies
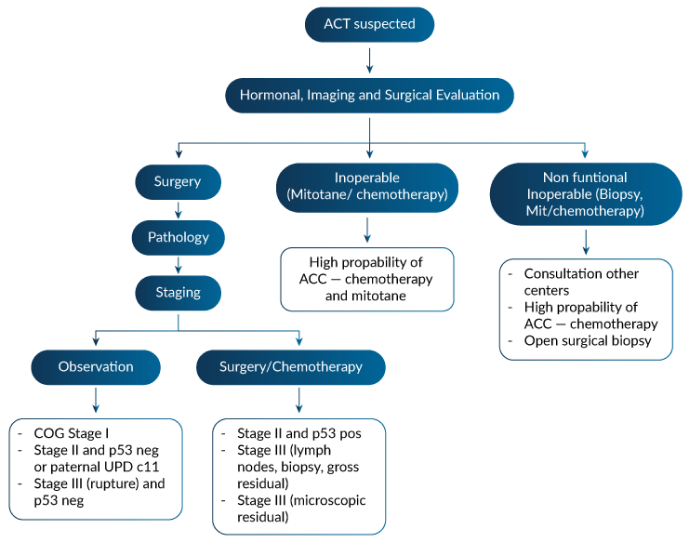
Dissection of lymph nodes is practiced under the justification to reduce recurrence (i.e., it could be prophylactic) or to follow the staging protocol, specially in the TNM system. Given the worse outcome demonstrated in ARARO332, it seems controversial or with uncertain clinical benefit.
Dr. Ribeiro compared the criteria and outcome of 3 different registries: ARARO332 , the French Registry and the Italian Registry surgical protocols (including lymph nodes dissection) associated with adjuvant therapies, considering the large difference between very low germline TP53 mutations prevalence (<30% in Europe) and very high in Brazil (>90%) and age differences.
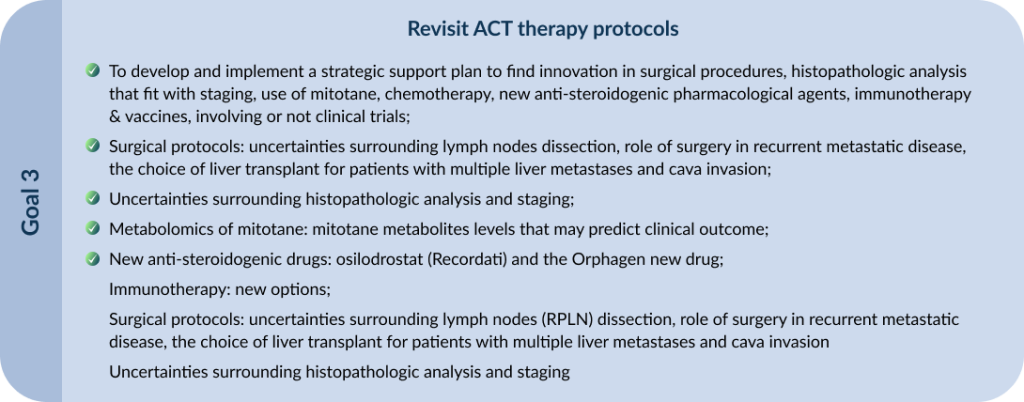
Based on that, we envision a new preposition for stage II criteria and other changes in management, both presented and discussed initially in Varandas Meeting by Raul C. Ribeiro, which would be soon presented in a manuscript to be shared and discussed in this consortium. This manuscript will have the co-authorship of all consortium members, in a position according to dedication spent in the manuscript preparation and number of stage 2 patients from the same institution.
All clinical treatment responsibilities for individual patients or research subjects shall remain under the sole control and be the sole responsibility of local treating physicians and researchers.
Evidence for surgery upfront
Good evidence of surgery upfront, particularly in localized disease
Opportunities
- Surgery instead chemotherapy for children with microscopic residual disease
- Neo-adjuvant chemotherapy in large tumors (anticipation of difficult resection, vascular extension)
- Role of surgery in recurrent and metastatic disease
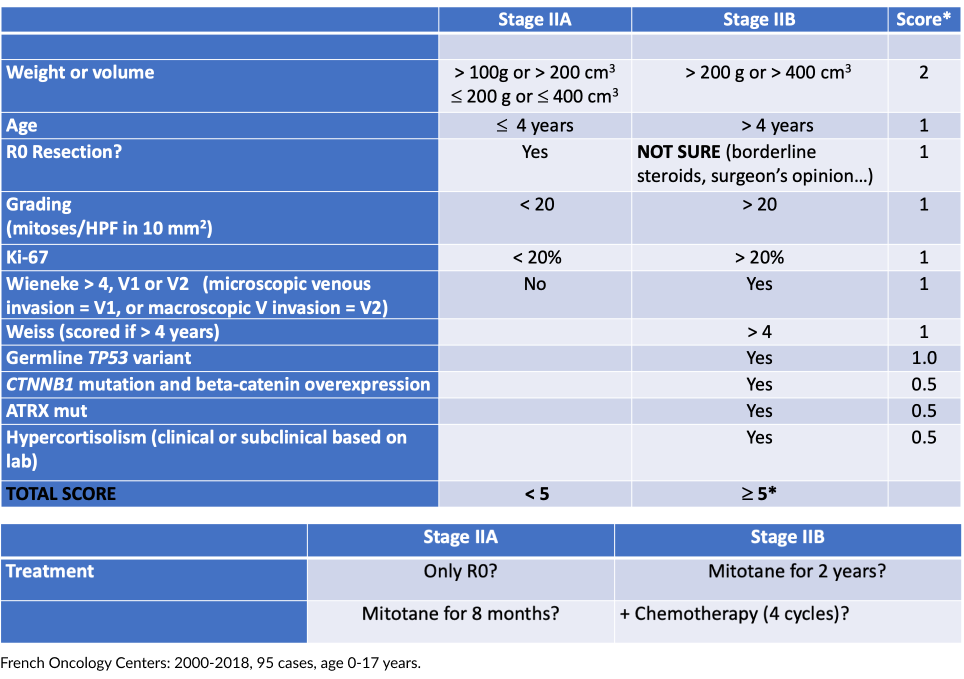
Stage II outcome:
According to staging, weight/volume, grading, vascular/capsular invasion, surgery plus RPLND or only surgery, neoadjuvant conventional.
This will be a manuscript coauthored by all participants of the consortium (data collected until November/23) reviewing retrospective stage II cases of each Clinical Center from the last 10 years
Ethics Committee (EC): To be firstly submitted to Pequeno Príncipe Hospital’s EC until July/23 and later validated/approved by each Institutional EC
Bonald, Mara Pianovski and Raul will be collecting the data and performing the initial reviews
Almost all Stage II ACT in Southern and Southeastern Brazil are germline TP53 (80 to 95%) and the majority is TP53 p.R337H
Therefore, this parameter alone is not sufficient to stratify
IIA (5-20%) from IIB (all germline TP53) (80-95%).
Furthermore, in Paraná state: 90-95% are p.R337H
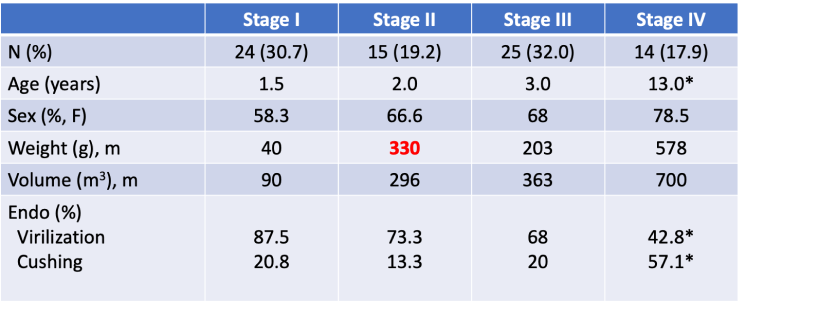
Outcome—Stage II disease (16 patients)
- 5-year OS of 93.3 and 5-year PFS of 78.8%
- One patient received adjuvant mitotane because of high tumor volume; it was stopped after 21 months due to neurological intolerance. The patient had a local relapse 4 years later and treated surgery (R0), and mitotane for a total of 8 years.
- Another patient developed lung metastasis after 4 months and subsequent liver metastasis after 15 months. The patient underwent complete surgical resection of metastases, combined with conventional chemotherapy and mitotane for 9 years.
- One patient died from an unrelated cause.
French cohort germline TP53 variants 22%
Expert Opinion—French Group (2)
- Mitotane duration of treatment is unknown. Suggestion to use for 2 years, because the majority of relapses occur during this time.
- The objective role of mitotane is difficult to ascertain, and given the effect of other therapies, to extend its duration if it is well tolerated.
- The toxicity of mitotane is a major limitation. There is currently no evidence supporting the use of prolonged administration of mitotane.
- First-line therapy systemic therapy consists of etoposide, doxorubicin, and cisplatin, in combination with mitotane.
- Second-line therapies have been used, with a very low impact on survival. The failure of second-line therapy should be considered palliative.

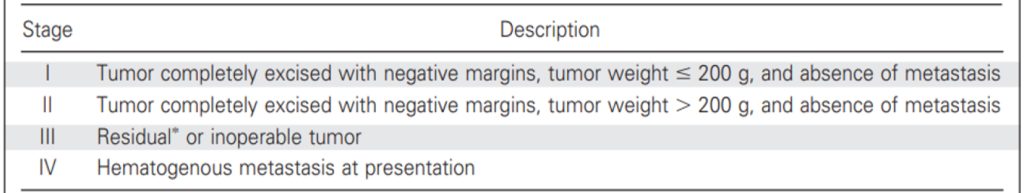
Italian ACT Registry—Retrospective Analysis
- Patients < 19 years
- Stage I: Radical excision and tumor volume < 200 cm3 and subsequent normalization of hormonal blood levels
- Stage II: Radical excision, microscopic residual tumor, or tumor volume ≥ 200 cm3 and subsequent normalization of hormonal blood levels
- Stage III: Excision with macroscopic residual)
- Stage IV: Metastases
Children’s Oncology Group ARAR0332 Trial
- Prospective single-arm risk-stratified interventional study.
- Opened in September 2006 and closed in May 2013.
- Patients < 22 years, newly diagnosed, previously untreated adrenocortical carcinoma (central review, Weiss and Wieneke)
– Can RPLND improve outcome for patients with large (stage II) tumors?
– Can intensive chemotherapy (MTT/CED) improve outcome for patients with advanced (stage III and IV) disease?
IPACTR cohort germline TP53 variants 80%
Age, staging and clinical signs of ACT cases enrolled in ARAR0332
Treatment according to stage in ARA0332

Analysis of the Primary Results
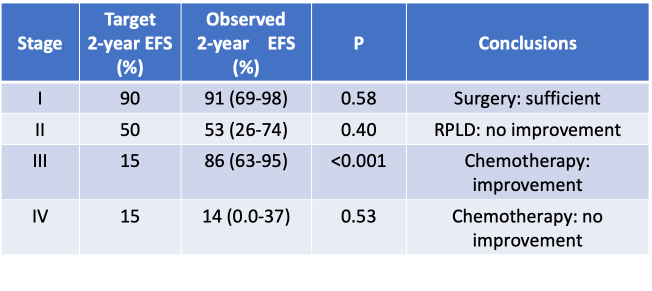
Feasibility of Therapy Delivery
- Toxicity was assessed by NCI CTCAE (version 4)
- Patients who had mitotane stopped because of toxicity counted as feasibility-event (MFE).
- Patients who had chemotherapy stopped because of toxicity counted as a chemotherapy feasibility-event (CFE).
Results of the toxicity/feasibility analysis
- Among 38 evaluable patients
– Four had an MFE probability of 10.5% (2.9%-24.8%)
– Twelve had a CFE probability of 31.6% (17.5%-48.7%) - Based on the study design, the chemotherapeutic regimen was not feasible and that further modifications would be required to improve tolerance.

Expert Opinions
- Surgery remains the mainstay treatment; the value of retroperitoneal lymph node dissection has not been defined.
- For patients with relapsed stage II and stage III, chemotherapy is associated with an improved outcome but is poorly tolerated.
- Innovative approaches are necessary for stage IV disease.
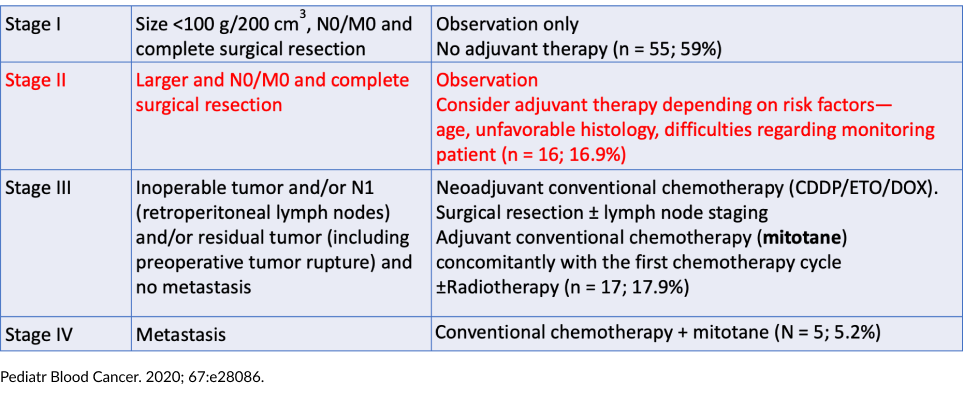
Stage I
- Five mitotane
- One radiotherapy
- One chemotherapy
Stage II (N total = 15)
- 13 mitotane
- Two chemotherapy
- Local radiation

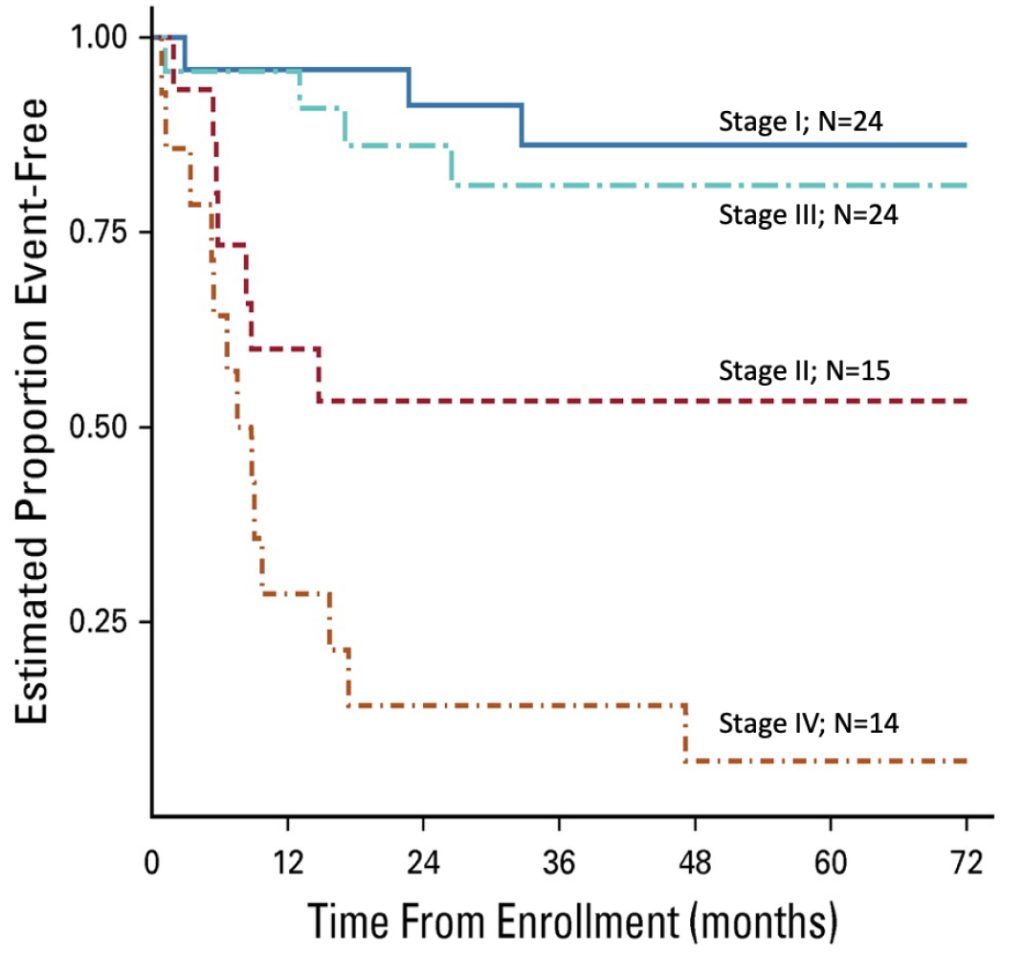
Event-free probabilities for 78 patients enrolled on ARAR0332